A drop of blood to save the world
06 April 2022
Bottom Line
Exact Sciences is the current leader in colorectal cancer screening with its stool-based Cologuard test. Two readouts on blood-based screening tests for colorectal cancer are due this year and will be pivotal in supporting FDA approval and market adoption. These data can potentially disrupt the current screening test market for this indication.
Liquid biopsy is an emerging approach that might change the cancer screening landscape. Colorectal cancer will be only the beginning. Our portfolios are currently exposed to both stool and blood-based approaches, and we are closely monitoring the developments in this field.
A Crucial Year For The Colorectal Cancer Screening Market
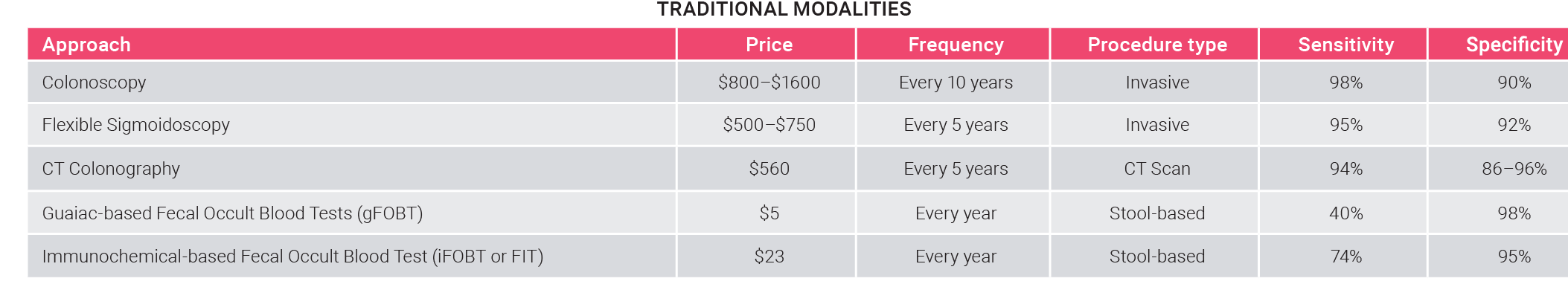
Current screening modalities fall short of perfection
Colorectal cancer is one of the most common and preventable types of cancer. Early detection, through screening tests, leads to a lower mortality rate, but the high invasiveness and complexity of current approaches limit their adoption.
- Despite recommendations, 1 in 3 people between 50 and 75 years old do not undergo colorectal cancer screening.
Non-invasiveness is crucial
Exact Sciences’ stool-based test, Cologuard, is currently the leading non-invasive colorectal cancer screening product. The use of blood tests as an alternative is still in its infancy.
- Exact is developing Cologuard 2.0, designed to improve efficacy.
- The only FDA-approved blood test still exhibits poor sensitivity and specificity.
New blood-based tests may soon reshape the screening market
While Exact is trying to consolidate the leading position of its stool-based test, its main rivals, Guardant Health and Freenome, are conducting clinical trials for their blood-based approaches, which are set to benefit from the already established reimbursement coverage for blood-based FDA-approved tests.
- Guardant Health and Freenome plan to present clinical data from large pivotal trials by end-2022. Guardant also expects to submit its test to premarket approval (PMA).
- Blood-based tests require an easier sample collection compared to stool-based tests and might increase compliance rates (Cologuard’s compliance rate is around 60%)
The Leading Modality Is Too Invasive
Earlier detection increases the chances of survival
Colorectal cancer is the third most common cancer type and a leading cause of death. If detected early, the disease is highly curable and may significantly increase the patients’ five-year survival rate.
- Five-year survival rate for stage I colorectal cancer is around 92% vs. 11% for stage IV.
An expanding addressable market
People over 50 years old in the U.S. (~100mn people) are considered at average risk for colorectal cancer and are recommended by the USPSTF to undergo screening. Last year the guidelines were updated to include the 45–49-year-old population.
- The new guidelines added ~19mn people to the addressable market of colorectal cancer screening.
Drawbacks of current screening modalities
Today’s traditional screening modalities (tissue-based testing, colonoscopy, Fecal Immunochemical Test) are too invasive or require a lot of preparation before the procedure, leading to poor compliance rates. The most invasive modality, colonoscopy, is also the most reliable and currently dominates the market.
- The compliance rate of traditional screening modalities is around 60%.
- Colonoscopy owns the lion’s share, with ~75% of the market, followed by stool-based screening (used in ~20% of patients) and blood-based screening (with a 6% penetration rate).
- Blood or stool-based tests could be used as first-line screening for asymptomatic people, where only those who are positive will undergo a colonoscopy.
An Upcoming Game-Changer
Liquid biopsy is eyeing colorectal cancer screening
Liquid biopsy companies are turning their attention to colorectal cancer screening as their next indication. Guardant Health and Freenome are currently conducting clinical trials and expect to share their results by end-2022. A high sensitivity and specificity blood test would be a game-changer.
- ~20% of screening volume is expected to shift from colonoscopy toward blood-based tests over the next five years.
- Guardant’s initial sensitivity and specificity are around 91% and 94%, respectively.
An approach with a larger addressable market
Unlike the Cologuard test, which is performed every three years, blood-based tests are performed annually. This approach could threaten Exact’s market position by reducing its addressable market.
- Blood-based tests target an annual market opportunity of around $25bn in the U.S., assuming a test price of ~$200.
- Exact is developing its blood-based test, and the study will first report this year, but it still lags behind the competition.
Reimbursement has been established
Medicare recently established coverage for blood-based colorectal cancer screening tests. Only FDA-approved tests will be candidates for reimbursement.
- Last year, the CMS implemented the national coverage decision (NCD) for blood-based colorectal cancer screening tests that comply with specific requirements.
- Guardant is conducting a 12,750 patients' pivotal trial (ECLIPSE study) to support the premarket approval (PMA) submission to the FDA by 2H 22.
A Seismic Shift In MedTech?
Catalysts
- The first launch to test the waters. Guardant will launch its first LDT colorectal screening test, Guardant Shield, in 1H22, ahead of FDA approval. This launch will show whether the medical community welcomes the approach.
- Clinical readouts. Guardant Health and Freenome will present data from large pivotal trials in 2022, supporting the adoption and sparking confidence in blood-based approaches.
- FDA approval of the first high-performance test. Guardant will submit the FDA premarket approval (PMA) of Guardant Shield in 2H22. FDA-approved high-performance tests will benefit from Medicare coverage, driving adoption.
Risks
- Stool-based tests are fighting back. Cologuard 2.0 is being tested under a large pivotal study, expected to read out around the end of 2022. Good results may undermine the adoption of emerging blood-based tests
- Disappointing clinical readouts. Blood-based tests are expected to publish new clinical data this year. Poor test performance will lead to a lack of confidence in the potential of the liquid biopsy for future indications.
- Poor acceptance and awareness. Doctors’ lack of awareness and skepticism around emerging approaches might lead to longer adoption curves.
Source:
OncoLogic Advisors
Companies mentioned in this article:
Epigenomics (ECX GR), Exact Sciences (EXAS US), Freenome (not listed), Guardant Health (GH US)
Explore:
Disclaimer
This report has been produced by the organizational unit responsible for investment research (Research unit) of atonra Partners and sent to you by the company sales representatives.
As an internationally active company, atonra Partners SA may be subject to a number of provisions in drawing up and distributing its investment research documents. These regulations include the Directives on the Independence of Financial Research issued by the Swiss Bankers Association. Although atonra Partners SA believes that the information provided in this document is based on reliable sources, it cannot assume responsibility for the quality, correctness, timeliness or completeness of the information contained in this report.
The information contained in these publications is exclusively intended for a client base consisting of professionals or qualified investors. It is sent to you by way of information and cannot be divulged to a third party without the prior consent of atonra Partners. While all reasonable effort has been made to ensure that the information contained is not untrue or misleading at the time of publication, no representation is made as to its accuracy or completeness and it should not be relied upon as such.
Past performance is not indicative or a guarantee of future results. Investment losses may occur, and investors could lose some or all of their investment. Any indices cited herein are provided only as examples of general market performance and no index is directly comparable to the past or future performance of the Certificate.
It should not be assumed that the Certificate will invest in any specific securities that comprise any index, nor should it be understood to mean that there is a correlation between the Certificate’s returns and any index returns.
Any material provided to you is intended only for discussion purposes and is not intended as an offer or solicitation with respect to the purchase or sale of any security and should not be relied upon by you in evaluating the merits of investing inany securities.
.png)